A study is a clinical research according to the MDR definition if the safety and/or performance of a medical device (instrument, device, software, reagent, material,...) are assessed using data derived from study participants.
On 26 May 2021, the European Medical Device Regulation (EU) 2017/745 (MDR) came into force. The MDR introduces a major update to the regulatory framework in the European Union and brings several changes to the scope of clinical studies to be submitted for approval, the initial submission processes and their substantial modifications, the content of the submission file and safety reporting.
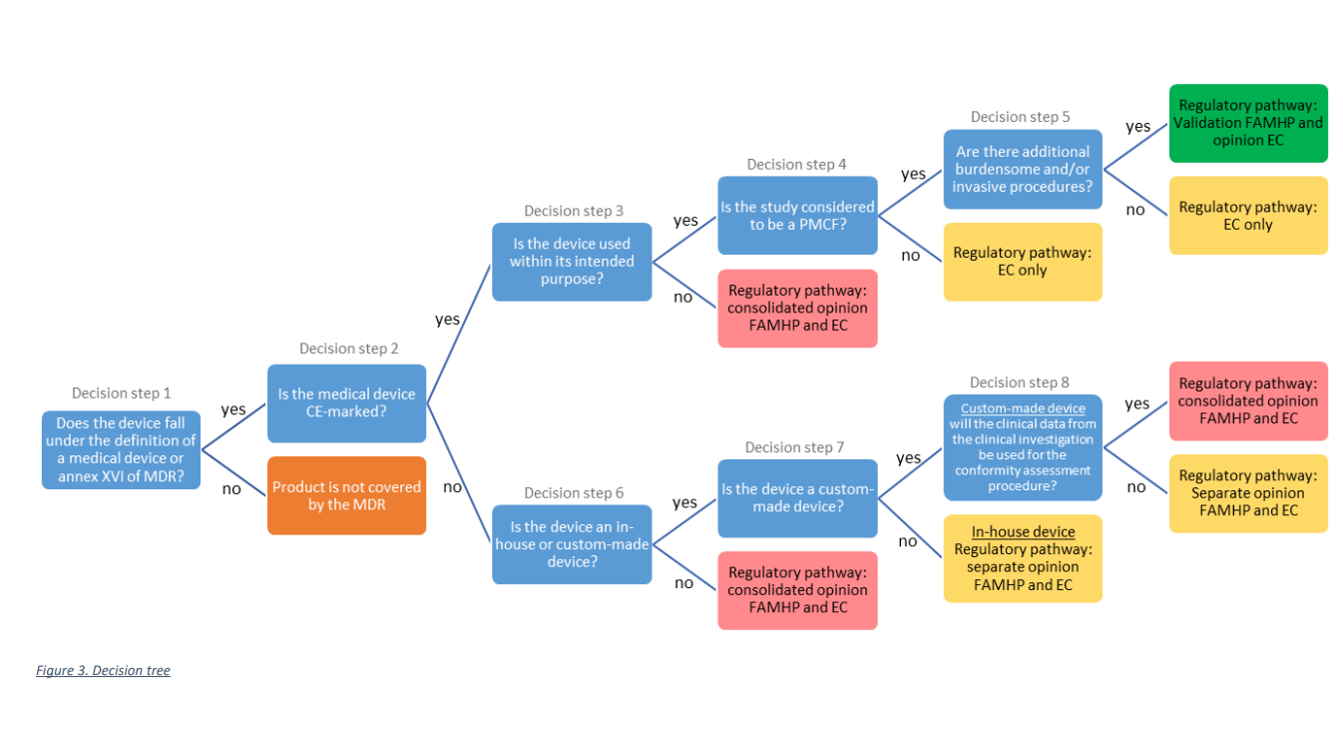
As can be seen from the decision tree below, the study needs to be submitted to the appropriate regulatory pathway, depending on the type of medical device used in the study and the type of study.
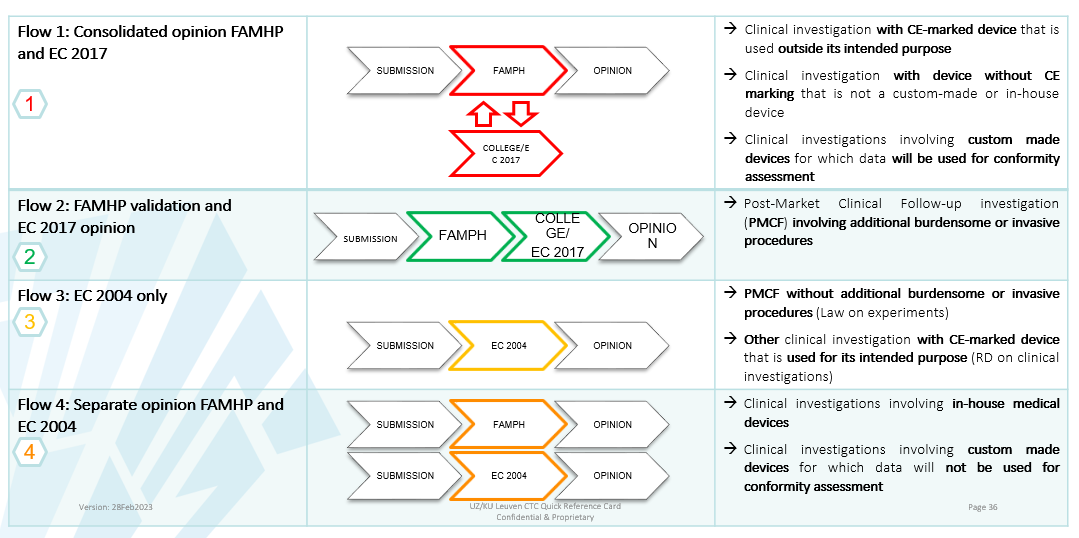
Different flows MDR
- More useful info on clinical research is available on the webpage of the FAMHP:
-
Any instrument, apparatus, appliance, software, implant, reagent, material, or another article can be a medical device. An app, therefore, can also be a medical device. The extensive definition of medical device is given in article 2 of the MDR.
The determination of whether something is a medical device is based on the intended (medical) purpose. The intended purpose describes what the product is designed to do.
On this website, you can do a quick scan to determine if a device or software should be qualified as a medical device: https://cetool.nl/en/medical-device