A performance study involves the evaluation of an in vitro diagnostic medical device (IVD) to determine or confirm its analytical or clinical performance. Analytical performance refers to the ability of an IVD to correctly detect or measure a specific analyte. Clinical performance refers to the ability to deliver results that are clinically relevant.
On May 26, 2022, the European Regulation (EU) 2017/746 on In Vitro Diagnostic Medical Devices (IVDR) came into force. The IVDR introduces a major update of the regulatory framework in the European Union and brings about several changes to the scope of performance studies, clinical studies with in vitro medical devices, that must be submitted for approval, the submission processes for initial application and substantial modifications, submission dossier contents and safety reporting.
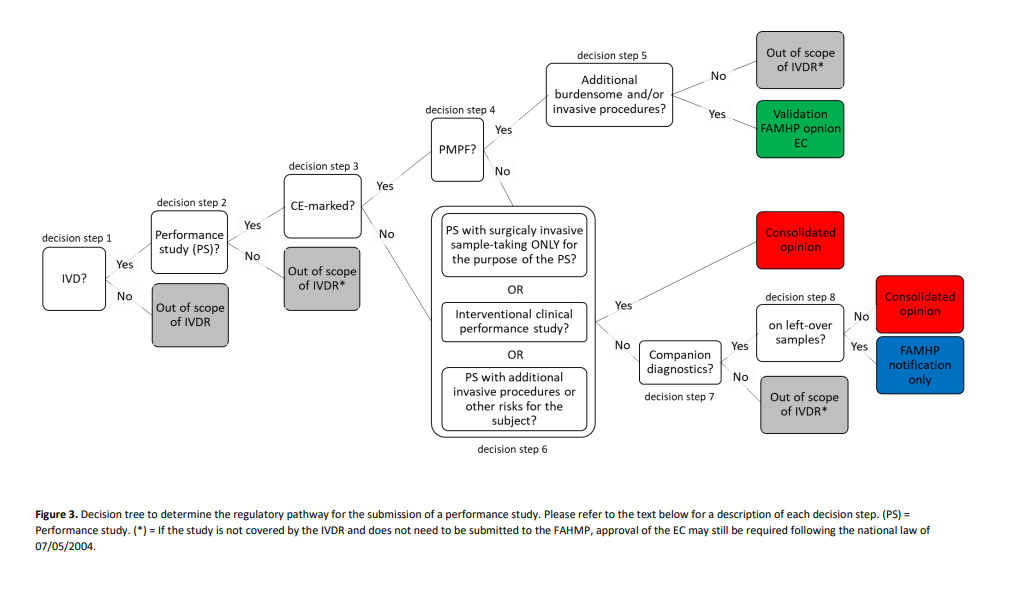
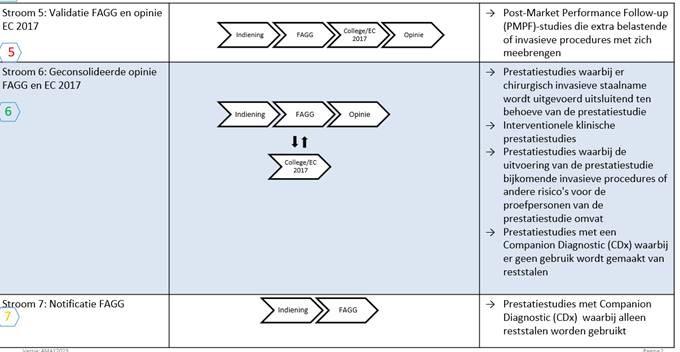
As can be seen from the decision tree below, the study needs to be submitted to the appropriate regulatory pathway, depending on the type of performance study.
More useful info on research within the scope of the IVDR is available on the webpage of the FAMHP; https://www.fagg-afmps.be/nl/MENSELIJK_gebruik/gezondheidsproducten/medische_hulpmiddelen_hulpstukken/klinische_evaluatie/inhoud_aangiftedossier